Novel Drug Combination for Malignant Pleural Mesothelioma Approved by FDA
Cancer one of the top diseases that affecting mankind across the globe, characterized by abnormal cells divide uncontrollably and destroy body tissue. Several kinds of cancers linked with different organs of the body like lungs, brain, bone and heart etc.
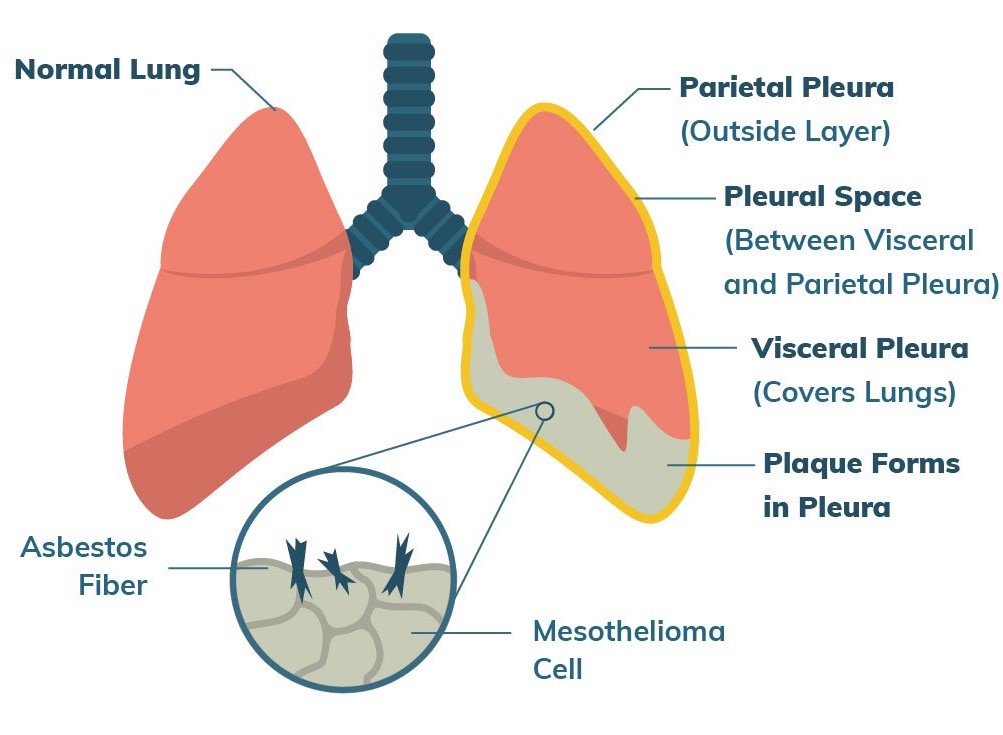
Malignant Pleural Mesothelioma (MPM), is one of the rarest cancers observed with cell linings of lungs, stomach and heart. It primarily triggers its action on lungs and caused by inhaling asbestos fibers. The symptoms associated are chest pain, cough and shortness of breath. The reported cases of MPM are less than 20, 000 per year in the USA. MPM is treated with chemotherapy, surgery and radiation therapy like rest of the cancers.
In 2004, FDA approved Pemetrexed in combination with cisplatin for the indication of MPM. After a long gap of 16 years and on October 2, 2020 the USFDA has approved Bristol-Myers Squibb’s combination of two drugs, Nivolumab and Ipilimumab for treatment of MPM. The first-line indication is for adults to treat MPM that can’t be taken out with surgery.
Both the drugs, Nivolumab (Opdivo) and Ipilimumab (Yervoy) are monoclonal antibodies and acts through activation of T- cell function, essential for controlling tumor growth. However, the said combination does pose side effects like Dyspnea, Musculoskeletal pain and Immune mediated reactions.
A noteworthy point here is that the approval by FDA occurred 5 months ahead of the goal date!




